Each Gelucire is characterized by two numbers, the first referring to the nominal melting point of the base and the second to the HLB value Gelucires come in a variety of grades with different melting points (from 33 ºC to 65 ºC) and HLB values (from 1 to 14) Mumbai Gelucire50/13 Gelucire44/14 and PEG6000 were obtained as giftGelucire 50/13 MinOrder 0 FOB Price USD $ 0000/ 1Our services:ASupply sampleBThe packing also can be according the customers` requirmentCAny inquiries will be replied within 24 hoursDwe provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificateThe dissolution rate of EVR from the optimized solid dispersion (SD) composed of the drug, gelucire 50/13 and microcrystalline cellulose in a weight ratio of 1510, was markedly rapid and higher

Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
Gelucire 50/13 melting point
Gelucire 50/13 melting point-In another embodiment, the present invention discloses a solid pharmaceutical dosage form being tablets composed of BCS class II drug like TEL, in solid carrier/hydrophilic carrier in gelucire such as stearoyl polyoxyl32 glycerides NF (Gelucire® 50/13) having melting point 50° C and HLB value 13 with pH modifiers like sodium hydroxide, sodium bicarbonate, magnesium oxide, potassium hydroxide, meglumine, sodium carbonate, citric acid, tartaric acid, ascorbic acid, malic acid;The role of two carriers —Gelucire 50/13 and PEG — was evaluated to improve the characteristics of the dissolution of albendazole, an anthelmintic drug with very low solubility and dissolution rate (HME)— simplifies the dispersion of hydrophobic drugs into low melting point hydrophilic carriers as well as their processing into a



Enhanced Bioavailability Of Sirolimus Via Preparation Of Solid Dispersion Nanoparticles Using A Supercritical Antisolvent Process Abstract Europe Pmc
GLC 44/14 (Lauroyl polyoxyl32 glycerides, melting point 44 ℃, hydrophiliclipophilic balance 14) and GLC 50/13 (Stearoyl polyoxyl32 glycerides, melting point 50 ℃, hydrophiliclipophilic balance 13) was kindly provided by Gattefosse (Cedex, France)Other products, such as Gelucire® 48/16 and Gelucire® 50/13 are solids (higher melting points) and so are suitable also for preparation of granules and powders which can subsequently be filled into hard shell capsules or sachets Alternatively, the powders may be compressed into tabletsChemsrc provides Gelucire 14/44(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of Gelucire 14/44 are included as well
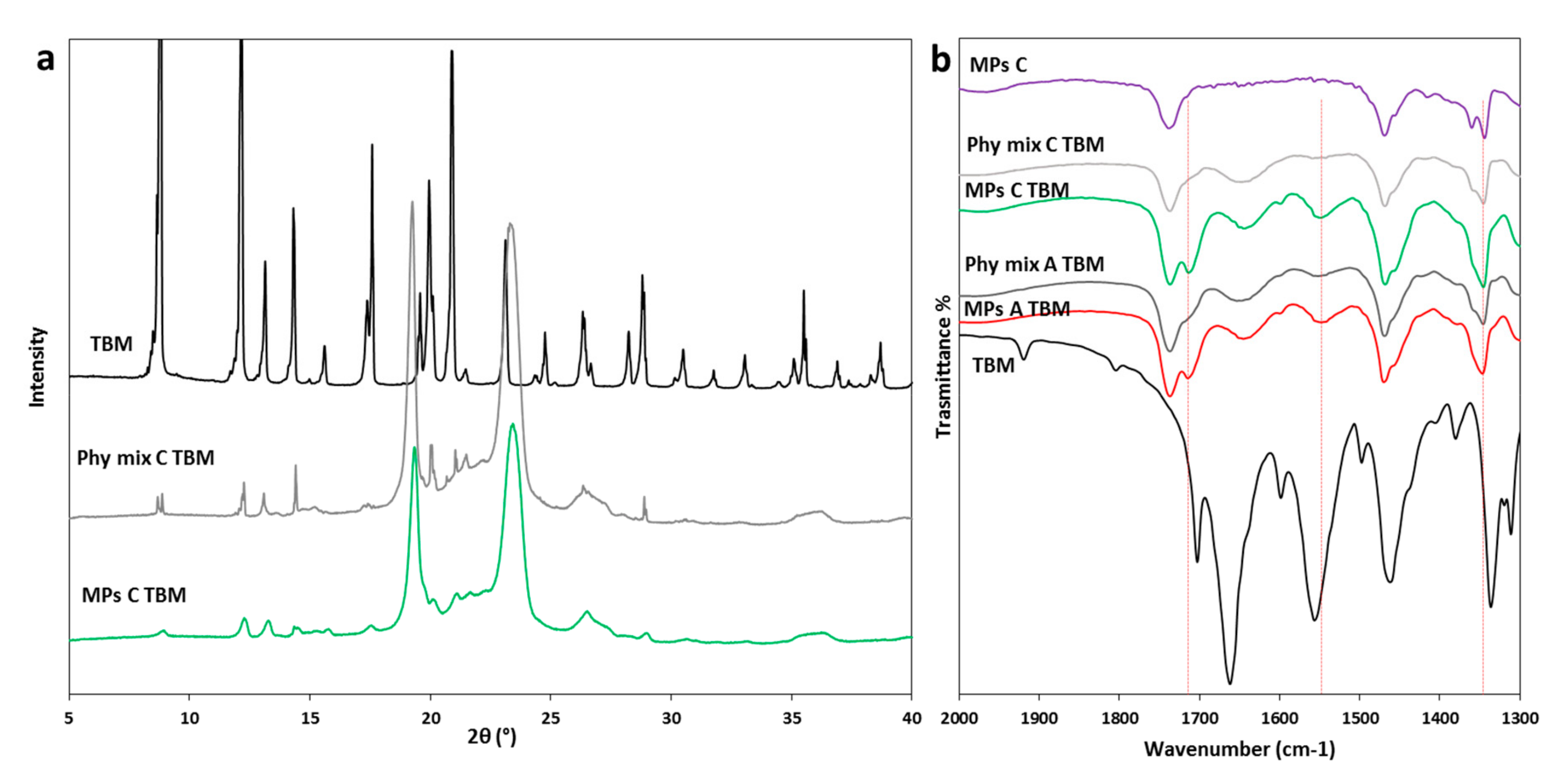
For the Gelucire 50/13, the peak at 1075 cm −1 can be assigned to ν(C OH) (7 cm −1 in comparison with Rozenberg's results), the peak at 1095 cm −1 assigned to ν(C O) trans (−9 cm −1) and the peak at 1112 cm −1 assigned to ν(C O) gauche (−14 cm −1)Gelucire® 48/16 and Gelucire® 50/13 are solids (higher melting points) and so are suitable also for preparation of granules and powders which can subsequently be filled into hard shell capsules or sachets Alternatively, the powders may be compressed into tablets Generally, selfemulsifying formulations provide a rapid (immediateGelucire is derived from mixtures of mono, di and triglycerides with PEG esters of fatty acids These are available with range of properties depending on their HLB and melting point range of 33 to 65 °C They have a wide variety of applications in oral and topical formulation
IND with PEG4000 or Gelucire 50/13, at 11, 12 and 14 weight ratio of INDdrug, were prepared by blending them by trituration for 10 min followed by sieving (500 lm) 222 Preparation of solid dispersion Solid dispersions (SDs) at various weight ratios were prepared by melting method IND was added to the molten base comprising PEG4000 orGLC 44/14 (Lauroyl polyoxyl32 glycerides, melting point 44 ℃, hydrophiliclipophilic balance 14) and GLC 50/13 (Stearoyl polyoxyl32 glycerides, melting point 50 ℃, hydrophiliclipophilic balance 13) was kindly provided by Gattefosse (Cedex, France)Gelucire 50/13 is an excipient composed of fatty acid (C16 and C18) esters of glycerol, PEG esters and free PEG It melts at approximately 50°C and has hydrophiliclipophilic balance (HLB) value of 13 6 , 7



Raman Spectra Of Gelucire 50 13 A And B In The Spectral Region From Download Scientific Diagram



Dissolution Profiles Of Atorvastatin And Atorvastatin Gelucire Formulae Download Scientific Diagram
Gelucire® 50/13 Gelucire® 50/13 is a well characterized excipient, supported by numerous publications and worldwide precedence of use including FDA IID listing It has similar surfactive properties to Gelucire® 44/14 However with a higher melting point and longer fatty acid chains it can have a release retarding effect when used at highDrug,gelucire 50/13 and microcrystalline cellulose in aweight ratio of 1510, was markedly rapid and higher than that from the drug powder and the market product (Afinitor®, Novartis Pharmaceuticals) in all dissolution mediums tested from pH 30 to pH 68 Jammula S et al 13studied the effect of Gelucire 50/13 on dissoAdditionally, changes in polymorphism may occur and alter dissolution rates during storage (eg, Gelucire 50/13—HLB 13, melting point 50°C) Also other excipients such as sucrose esters, which would be stable up to 140°C ( 312 ) and are available with a promising high HLB of 16, showed polymorphic transformation during storage after melting ( 173 )



An Investigation Into The Mechanism Of Dissolution Rate Enhancement Of Poorly Water Soluble Drugs From Spray Chilled Gelucire 50 13 Microspheres Qi 10 Journal Of Pharmaceutical Sciences Wiley Online Library



Enhanced Bioavailability Of Sirolimus Via Preparation Of Solid Dispersion Nanoparticles Using A Supercritical Antisolvent Process Abstract Europe Pmc
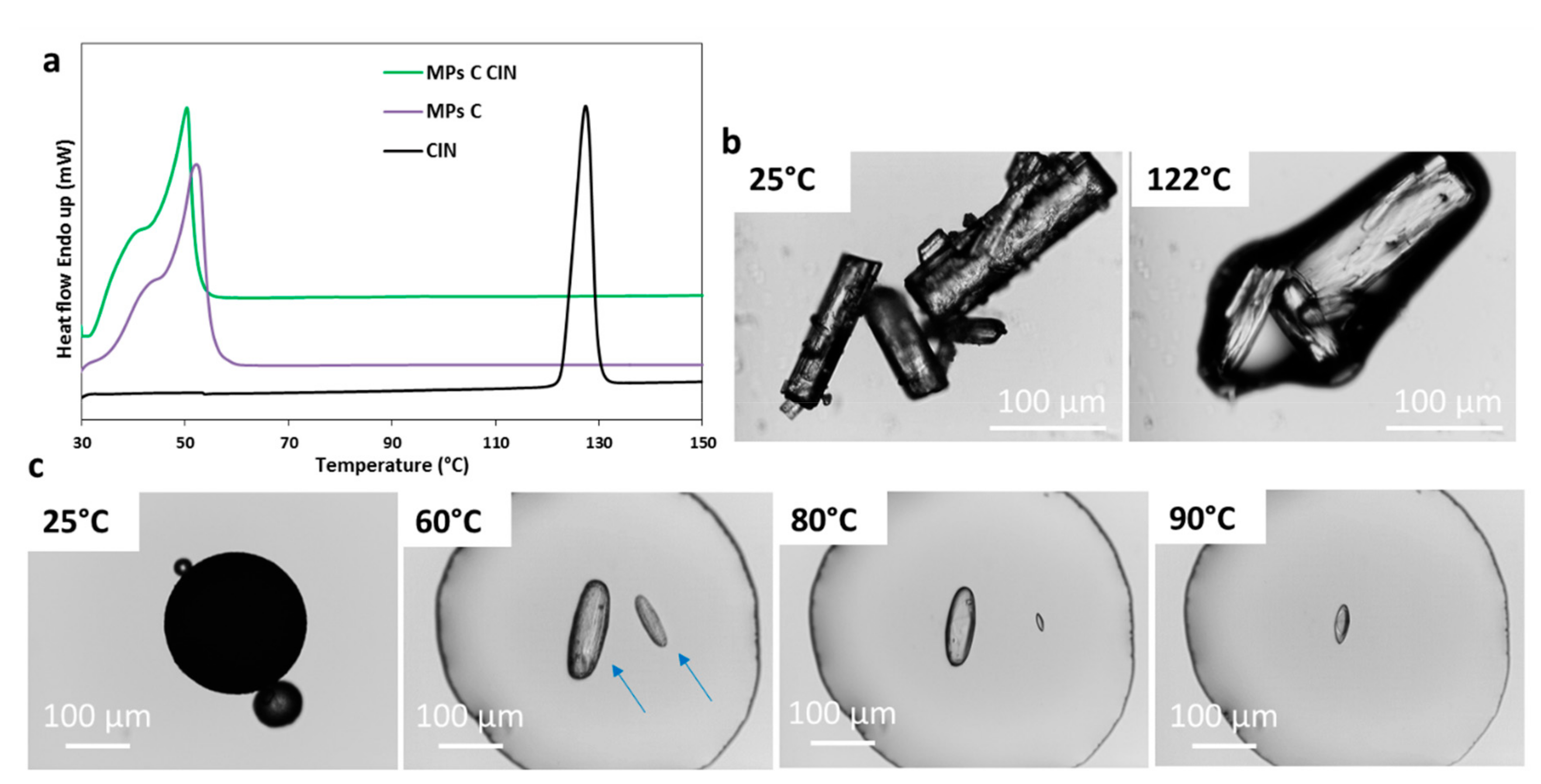
Fig 6 demonstrates thermograms of IND, Gelucire 50/13, their PMs and SDs The endotherm of Gelu displayed broad peak appeared at 4656 °C corresponding to its melting point with heat of fusion −160 J/g (Khan and Craig, 03)Other products, such as Gelucire® 48/16 and Gelucire® 50/13 are solids (higher melting points) and so are suitable also for preparation of granules and powders which can subsequently be filled into hard shell capsules or sachets Alternatively, the powders may be compressed into tabletsFor example, GELUCIRE 50/13 designates a melting point of approximately 50° C and an HLB value of about 13 to this grade of GELUCIRE



Ir Spectra Of Pure Drug And Solid Dispersion Of Gelucire 44 14 O And Download Scientific Diagram



Differential Scanning Calorimetry For Different Atorvastatin Formulae Download Scientific Diagram
The wide varieties of gelucires are characterized by a wide range of melting points from about 33°C to about 64°C and most commonly from about 35°C to about 55°C, and by a variety of HLB values from about 1 to about 14, most commonly from about 7 to about 14Gelucires are defined by their melting point/HLB value As discussed previously, even though most Gelucires have Tm > 37°C, (the most frequently used for SLN/NLC formulation up to now is Gelucire 50/13), their Tm after processing to colloidal dimensions is expected to be decreased, leading to only partially crystallized lipid matrixWO PCT/IN15/ INW WO WO WO WO IN W IN W IN W WO WO WO Authority WO WIPO (PCT) Prior art keywords pharmaceutical composition solid tel carrier gelucire Prior art date Application number



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Reported Literature On Gelucire 50 13 Download Scientific Diagram
42—46°C) and Gelucire® 50/13 (HLB 13, melting point 46—51°C) (Gattefossé, Saint Priest Cedex, France) were used All organic solvents were HPLC grade All other chemicals were analytical grade and doubledistilled water was used throughout the study The wide varieties of gelucires are characterized by a wide range of melting points from about 33°C to about 64°C and most commonly from about 35°C to about 55°C, and by a variety of HLB values from about 1 to about 14, most commonly from about 7 to about 14Legal notice Terms and condition © 21 Gattefossé People make our name



Epa1 Improved Fenofibrate Compositions Google Patents


Pdf Study On Mechanism For Amorphous Drug Stabilization Using Gelucire 50 13
DSC thermograms of FFA, GL 50/13, PL F127, and solid dispersion are shown in Figure 1 The DSC curve for untreated drug showed a sharp endothermic peak at 1353°C, which corresponds to its melting point indicating the crystalline nature of the drug (Fig 1E)Gelucire® 44/14 (melting point 44 °C, HLB=14), Gelucire® 50/13 as core material were manufactured as follows after heating Witepsol®, Ovucire®, Suppocire® or Gelucire® to °C above melting point, the molten material was admixed with theophylline monohydrate (the theophylline monohydrate content was either 30% or 60% (w/w)) andGelucire 50/13 and polyethylene glycol (PEG) 8000 were evaluated as low melting point solid dispersion carriers Neusilin US2 (magnesium aluminosilicate as a spraydried amorphous powder with high specific surface area, excellent flow, and good compressibility) was used as the surface adsorbent



Enhanced Bioavailability Of Sirolimus Via Preparation Of Solid Dispersion Nanoparticles Using A Supercritical Antisolvent Process Abstract Europe Pmc
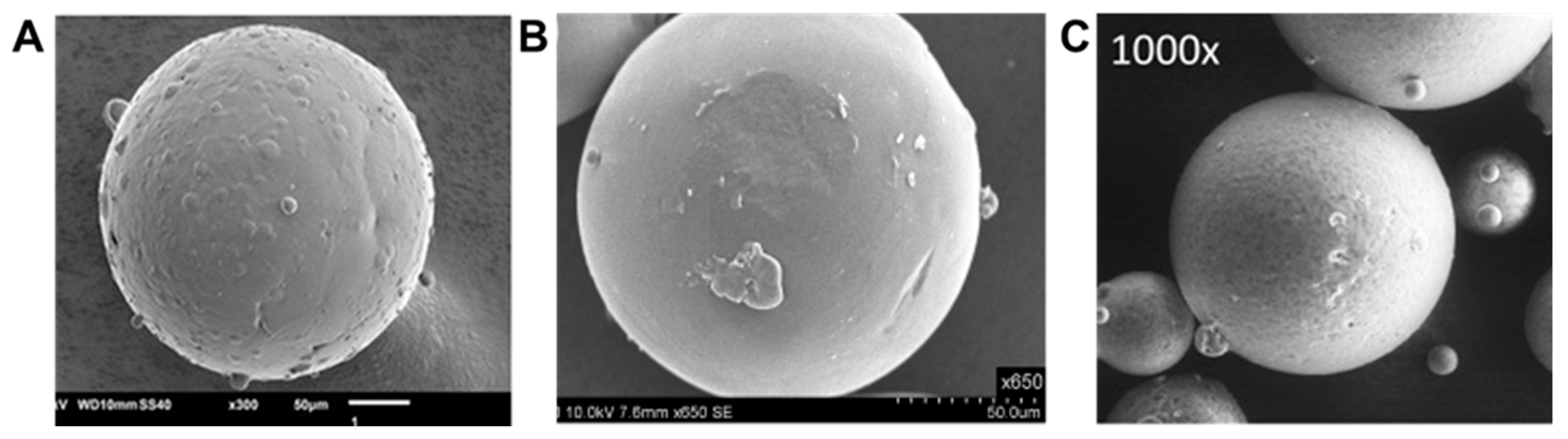


Molecules Free Full Text Spray Congealing An Emerging Technology To Prepare Solid Dispersions With Enhanced Oral Bioavailability Of Poorly Water Soluble Drugs Html
Gelucire 50/13 is a semisolid excipient with an HLB value of 13 and melting point of 500C Its hydrophilic property and low melting point makes it a good choice for use as carrier in preparation of solid dispersions by fusion method (Eloy et al, 12) Poloxamers are polyoxyethylenepolypropylene block14) and GLC 50/13 (Stearoyl polyoxyl32 glycerides, melt ing point 50 °C, hydrophiliclipophilic balance 13) was kindly provided by Gattefosse (Cedex, France)The melting points of curcumin and Gelucire®50/13 are 172 and 464°C, respectively As shown in Fig 3 , there are no changes in the main events, temperatures or intensities of the peaks for SD0, SD3 and SD6



Saxs A And Waxs B Diffractogram Of Gelucire 50 13 As A Function Of Download Scientific Diagram



Solid Dispersion As An Eminent Strategic Approach In Solubility Enhancement Of Poorly Soluble Drugs
Gelucires are characterized by a wide range of melting points, from about 33 °C to about 65 °C, and by a variety of hydrophilic and lipophilic balance (HLB) values of approximately 1–18 (10, 11) Gelucires with low HLB can be employed to decrease the dissolution rate of drugs, and ones with high HLB for fast release (12, 13)IND with PEG4000 or Gelucire 50/13, at 11, 12 and 14 weight ratio of INDdrug, were prepared by blending them by trituration for 10 min followed by sieving (500 lm) 222 Preparation of solid dispersion Solid dispersions (SDs) at various weight ratios were prepared by melting method IND was added to the molten base comprising PEG4000 orThese two numbers are the melting point of the base (varies from 33 °C65 °C) and the HLB value of the proportion of watersoluble, parts to fatsoluble in each gelucire (varies from 1 to 14)
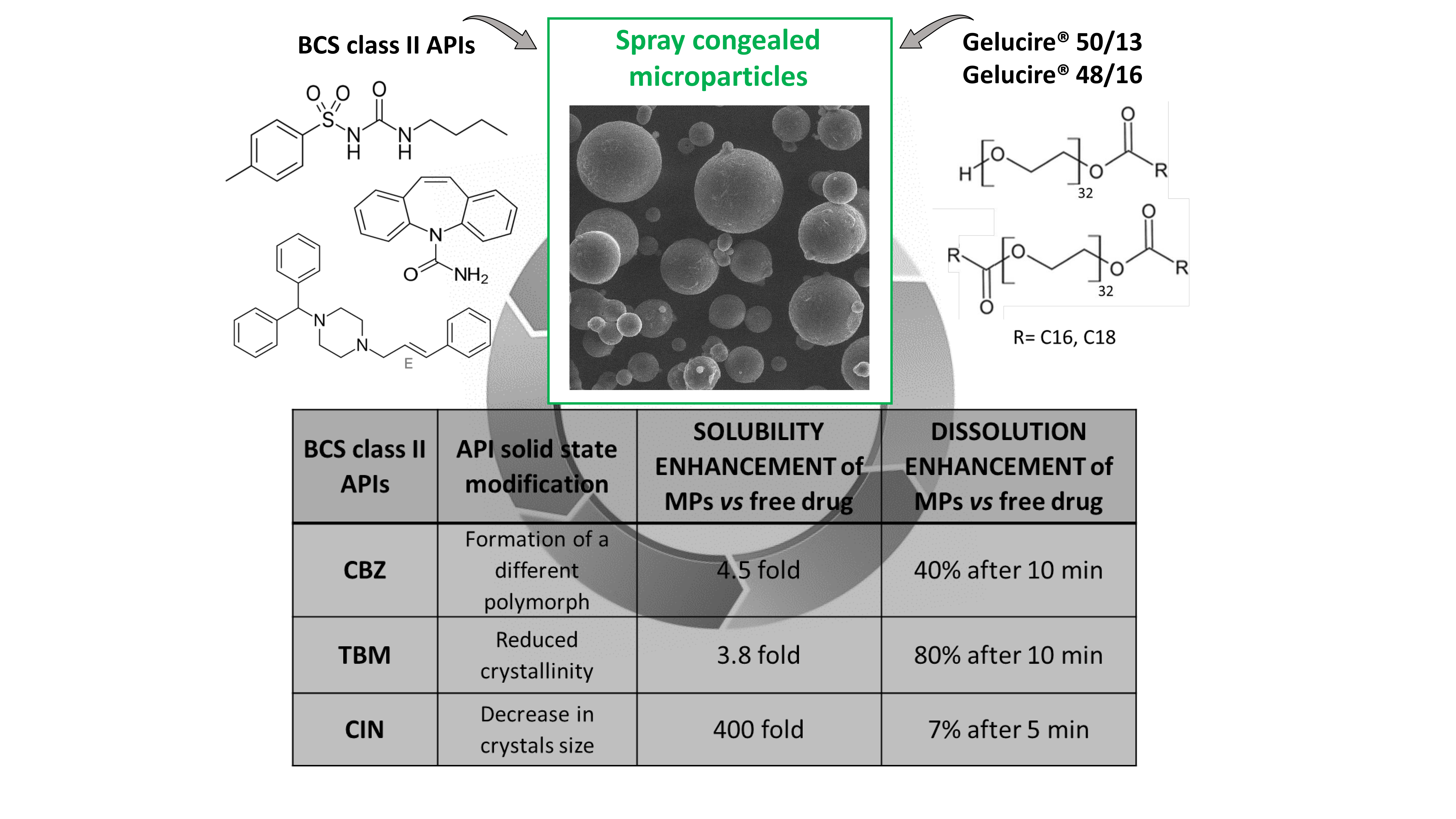


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html



Radiograph Powder Diffraction Patterns Etoricoxib Et Etoricoxib Gel Download Scientific Diagram
Each Gelucire is characterized by two numbers, the first referring to the nominal melting point of the base and the second to the HLB value Gelucires come in a variety of grades with different melting points (from 33 ºC to 65 ºC) and HLB values (from 1 to 14) Mumbai Gelucire50/13 Gelucire44/14 and PEG6000 were obtained as giftGelucire 50/13 is a semisolid excipient with an HLB value of 13 and melting point of 500C Its hydrophilic property and low melting point makes it a good choice for use as carrier in preparation of solid dispersions by fusion method (Eloy et al, 12) Poloxamers are polyoxyethylenepolypropylene blockVisit ChemicalBook To find more Gelucire 5013() information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes You can also browse global suppliers,vendor,prices,Price,manufacturers of Gelucire 5013() At last,Gelucire 5013() safety, risk, hazard



Formulation Of Immediate Release Pellets Containing Famotidine Solid Dispersions Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html
Initially, the excipient (Gelucirefi 50/13 and Gelucirefi 48/16 in di erent ratio) was heated up to a temperature of 5 C above its melting point The API (10% w/w) was added to the moltenThe role of two carriers —Gelucire 50/13 and PEG — was evaluated to improve the characteristics of the dissolution of albendazole, an anthelmintic drug with very low solubility and dissolution rate (HME)— simplifies the dispersion of hydrophobic drugs into low melting point hydrophilic carriers as well as their processing into aDrug,gelucire 50/13 and microcrystalline cellulose in aweight ratio of 1510, was markedly rapid and higher than that from the drug powder and the market product (Afinitor®, Novartis Pharmaceuticals) in all dissolution mediums tested from pH 30 to pH 68 Jammula S et al 13studied the effect of Gelucire 50/13 on disso



Gelucire 5013 Melting Point ただのゲームの写真



X Ray Powder Diffraction For Different Atorvastatin Formulae A Using Download Scientific Diagram
After classified by two numbers, the first referring to the complete dissolution of ALLO and gelucire 50/13 in approximate melting point of the base and the second ethanol sonicated the solution for minutes, and then to the hydrophilic lypophilic balance (HLB) value The solvent was evaporated under reduced pressure at nature and proportionGelucire 50/13 allows the formation of larger crystals than PEG, using both the chemical forms of the drug The release percentage of the drug from PEG6000/acidic diclofenac reaches 50% after few minutes in the most favourable case and appears to be dependent on the composition of the samples the more diclofenac is present as dissolved in theName Composition Hydrophilic–lipophilic Melting point ( C) Saponification value balance (HLB) (mg KOH/g) Precirol® ato 5 Mono, di and triglycerides of saturated C16 and 2 53–57 165–195 C18 fatty acids Gelucire® 50/13 Mono, di and triglycerides of saturated C8C18 13 46–51 67–81 fatty acids mono and difatty acid esters of PEG



Gelucire 50 13 Pharma Excipients
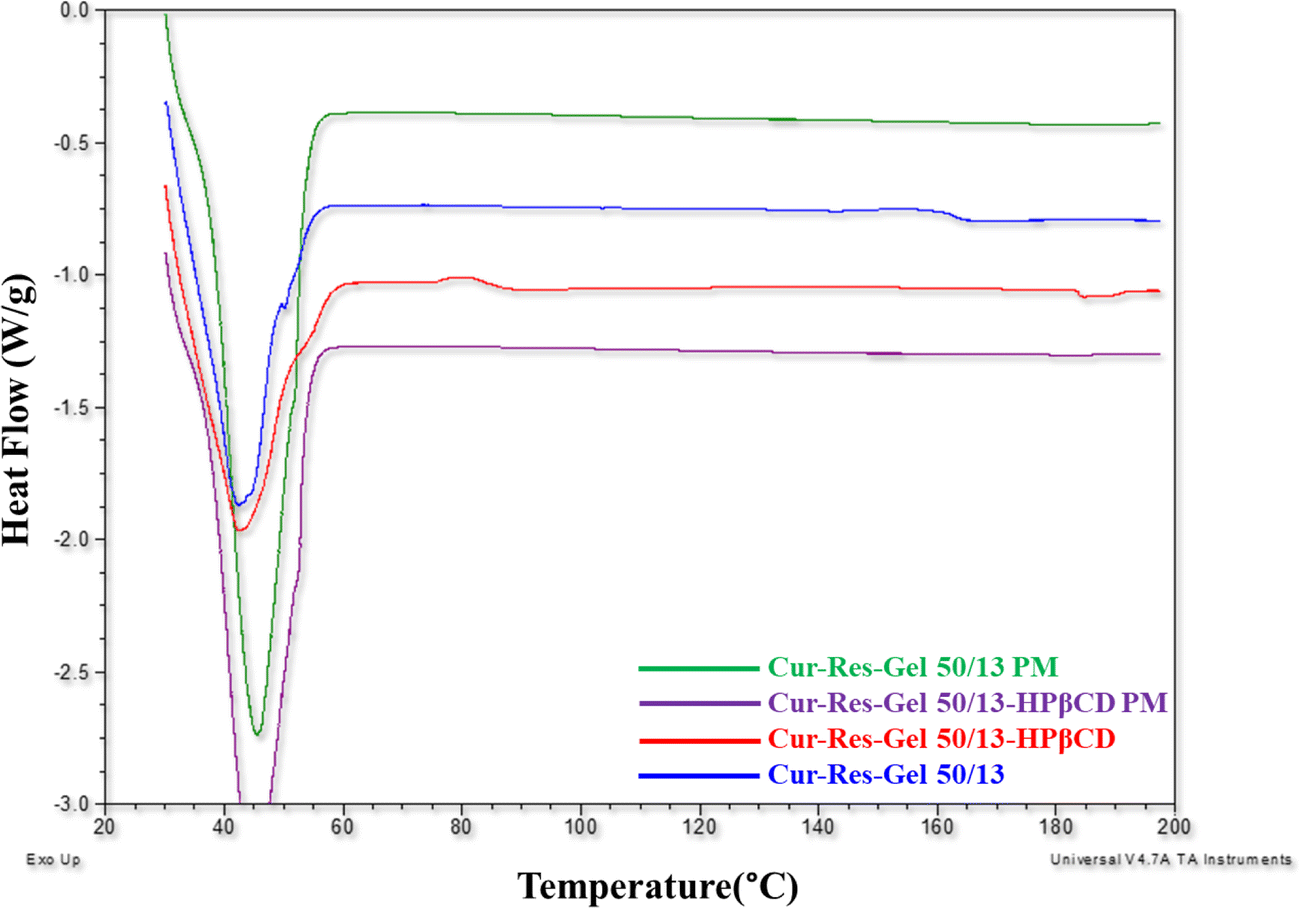


Figure 8 Preparation Characterization And In Vitro Evaluation Of Curcumin And Resveratrol Loaded Solid Lipid Nanoparticles Springerlink
WO PCT/IN15/ INW WO WO WO WO IN W IN W IN W WO WO WO Authority WO WIPO (PCT) Prior art keywords pharmaceutical composition solid tel carrier gelucire Prior art date Application numberVisit ChemicalBook To find more Gelucire 5013() information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes You can also browse global suppliers,vendor,prices,Price,manufacturers of Gelucire 5013() At last,Gelucire 5013() safety, risk, hazardChemsrc provides Gelucire 14/44(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of Gelucire 14/44 are included as well



Wo 08 A1 Hot Melt Micropellets The Lens Free Open Patent And Scholarly Search
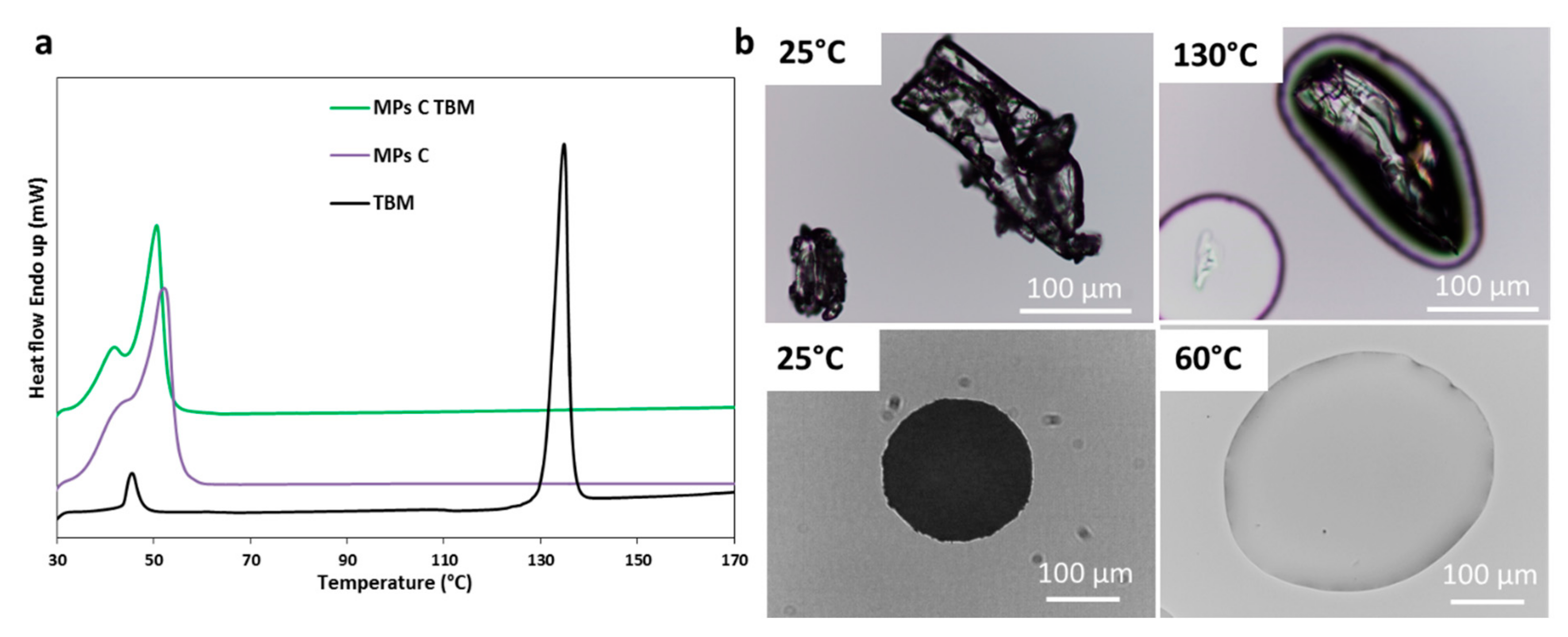


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html
For the Gelucire 50/13, the peak at 1075 cm −1 can be assigned to ν(C OH) (7 cm −1 in comparison with Rozenberg's results), the peak at 1095 cm −1 assigned to ν(C O) trans (−9 cm −1) and the peak at 1112 cm −1 assigned to ν(C O) gauche (−14 cm −1)



Pdf Preparation And In Vitro Evaluation Of Allopurinol Gelucire 50 13 Solid Dispersions
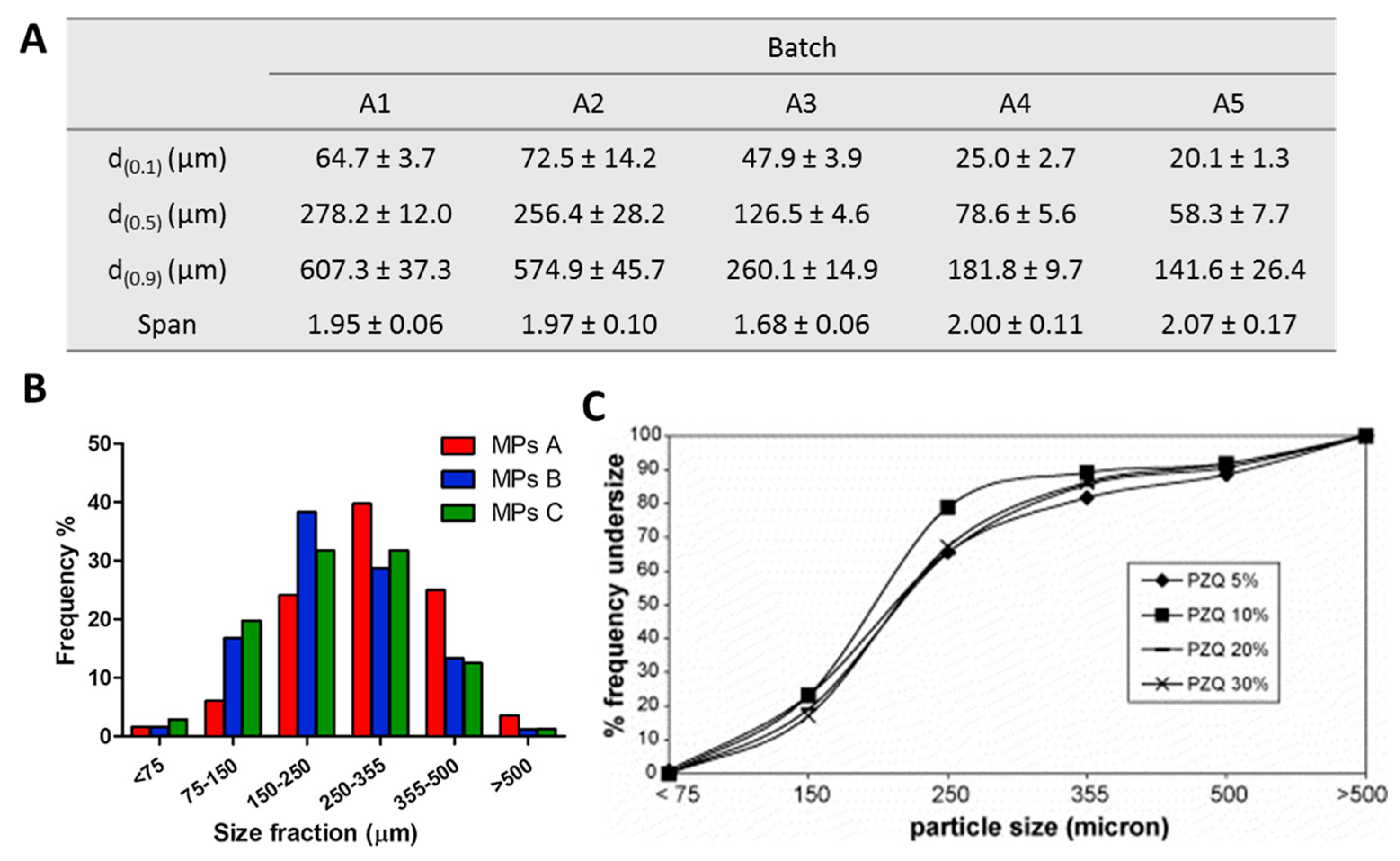


Molecules Free Full Text Spray Congealing An Emerging Technology To Prepare Solid Dispersions With Enhanced Oral Bioavailability Of Poorly Water Soluble Drugs Html



Epa1 Improved Fenofibrate Compositions Google Patents



Ir Spectra Of Gelucire 50 13 Fitted By Lorentzian Curves As A Function Download Scientific Diagram
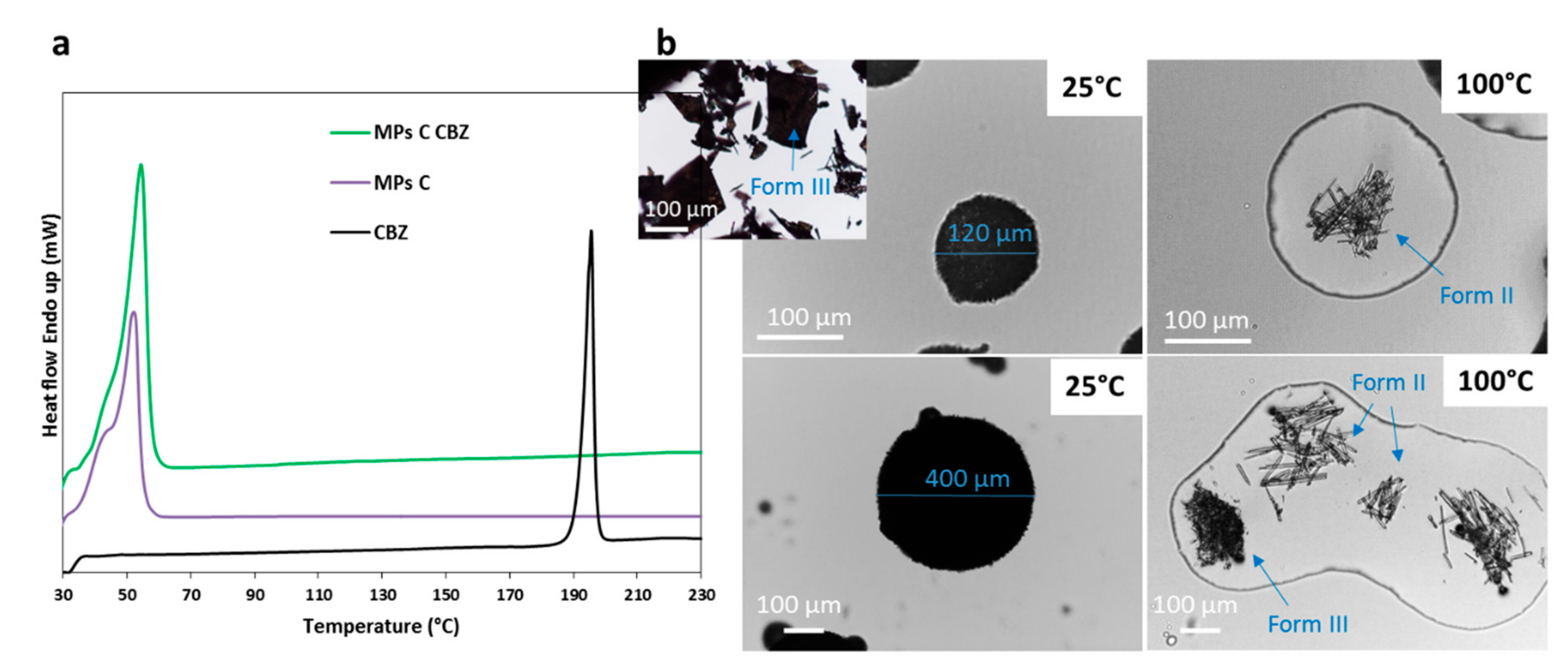


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html



Raman Spectra Of Gelucire 50 13 A And B In The Spectral Region From Download Scientific Diagram



Raman Spectra Of Gelucire 50 13 Fitted By Lorentzian Curves As A Download Scientific Diagram



Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems


Pdf Gelucire 44 14 Based Immediate Release Formulations For Poorly Water Soluble Drugs



Xrd Diffractograms For The System Acidic Diclofenac Peg6000 A Download Scientific Diagram


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html



Epa1 Improved Fenofibrate Compositions Google Patents



Pdf Development Of Solid Sedds Iii Application Of Acconon C 50 And Gelucire 50 13 As Both Solidifying And Emulsifying Agents For Medium Chain Triglycerides


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Reported Literature On Gelucire 50 13 Download Scientific Diagram



Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents



Pdf Improvement Of Solubility And Dissolution Rate Of Indomethacin By Solid Dispersions N Gelucire 50 13 And Peg4000


Reported Literature On Gelucire 50 13 Download Scientific Diagram



Designing Optimal Formulations For Hot Melt Coating Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub
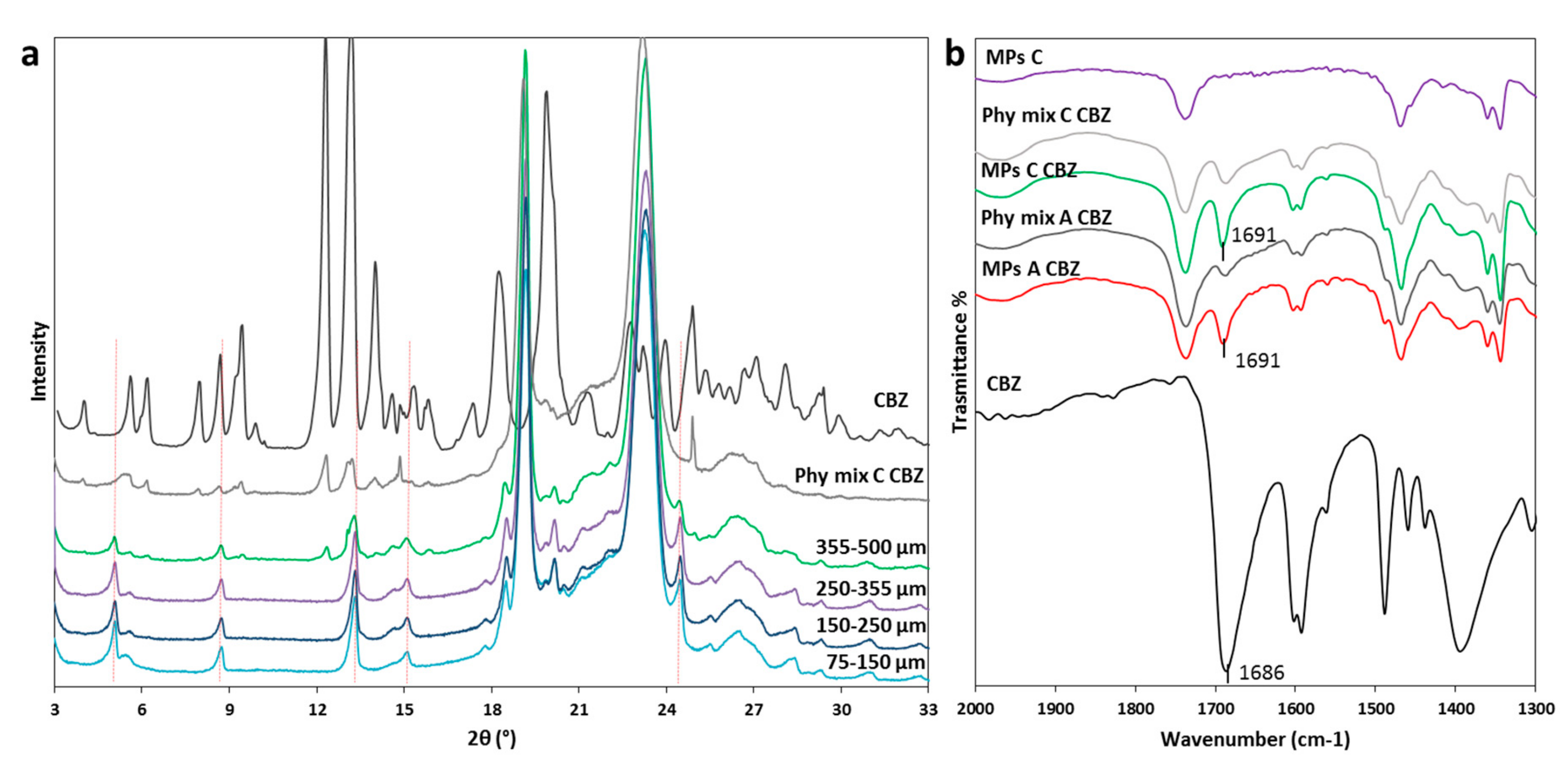


Pharmaceutics Free Full Text Different s Class Ii Drug Gelucire Solid Dispersions Prepared By Spray Congealing Evaluation Of Solid State Properties And In Vitro Performances Html


